



I also tried stacking a couple of the images with Picolay.

And here is the insect part, ostensibly from a bee. The piece is about 1/10 mm long.
An informal presentation of my journey from wide-eyed neophyte to owner of an affordable research microscope and all the bumps, bruises, pitfalls and yes, enlightenment, along the way. My perspective is that of a hobbyist and my goal is a layman's familiarity with the alien life of a water droplet.




I also tried stacking a couple of the images with Picolay.

And here is the insect part, ostensibly from a bee. The piece is about 1/10 mm long.
After a long winter of limited access to fresh specimens, my river is again open, at least near the banks. However, the winter was an enlightening season since it taught me much about protist cultures, how to maintain them and how to sample them.
It all started last fall when I filled a standard “fish bowl” with water, mud and a few plants taken from the small river I live on. I began with a relatively large collection of microscopic specimens, including some larger types like gammarus, copepods, ostracods, fairy shrimp and daphnia. The bowl was placed in an east facing windowsill where it received direct morning light. This little micro environment provided a large number of critters for observation but after some time the plants died and things slowed down. Luckily I had a 30 gallon tank that had gone wild and in cleaning it up I was able to add a large matt of algae to my bowl, again rejuvenating it.
 |
| The fish bowl on the extreme left was the container that started it all |
It was at that point I started a jar collection, seeding them with soil, water and algae from the fish bowl. These jars were “fed” with dried oak leaves, dead grasses from under the snow, vegetable scraps and various grains, cereals or even garden dirt. Most of these jars developed in different ways and provided a source of interesting, but limited, species over the winter. Another jar collection was started in a north facing window with none getting direct light. I had noticed the jars on the sunny sill were getting quite warm on clear days.
 |
| My north facing jars |
One jar in which I was doing a Walstad experiment developed an algae explosion that I used to feed several of the jars with larger inhabitants. What I found interesting was that some of my best microbe sources were the smallest containers I used, old 35 mm film containers. I even had a population explosion of several ciliate species under a cover slip on a slide I was keeping in a “wet” container to watch the development of snail eggs.
After providing a winter of enjoyment under the microscope the jars will soon be emptied back into the river, cleaned up and readied for some new, springtime populations.
I went back to the water sample from yesterday's post with the intent of getting better pictures to help with identifying this little critter. After several attempts I gave up and went the video route. I've tried going through several keys to ID her but I appear to have little talent in this area. Specimen is about 250 um long.
If you have any ideas please post them in the comments. Ive been told by an expert this specimen belongs to the Order Ploima, and possibly (slight) to the Genus Proales.
I have a small 35 mm film tube that I put a grain of rice into as a food source to see what would show up. It turned out to be quite rich in species, mostly small algae, ciliates and flagellates. However there are a number of larger ciliates, likely Stylonychia, and what I assumed were flatworms. The mode of locomotion was very reminiscent of a ciliated protist. Wrong! I think. Yesterday I saw no evidence of a corona but today I did, a very small and unobtrusive one.
Yesterday I was able to count them in the dozens but today I found only a few but with a lot of oval cysts (or eggs). So I'm assuming they are cysts (or eggs) that were the result of yesterday's rotifers. Not sure what changed, maybe a dropping food supply or unfavourable water conditions. Several were quite lethargic and there were also a few dead ones laying around.
 |
| 35 um long, 20 wide, smaller than most bdelloid eggs |

I've been keeping a slide with some snail eggs in a "wet" container so I can watch the eggs develop over time. Over a couple of weeks nothing has happened to the eggs but we have a secondary event going on I found interesting. There was a population explosion of ciliates, and only one kind that I thought were Stylonichia. Along with these were a large number of cysts which I believed were associated with the ciliates.So now I believe I know what Stylonychia cysts look like.
This cyst (left image) was a stack of 7 photos to get the whole object into focus.
 |
| Monoraphidium contortum, straight line length between tips is 18.4 um |
 | |||
| A few unidentified ones , small ones about 7um long and the raft of 4 about 10um long |
 |
| Genus Scenedesmus, 27 um long |
 |
| Unknown algae |
I isolated this large, contractile ciliate and chased it around the slide for some time. Even when 1/2 the water had evaporated from under the slide the specimen was still very active. No idea what this is. I have since been told this is actually a flatworm from Subphylum Catenulida.
 |
| In this view the specimen is 850um long |
 |
| In this contracted view the specimen is 480mm long |
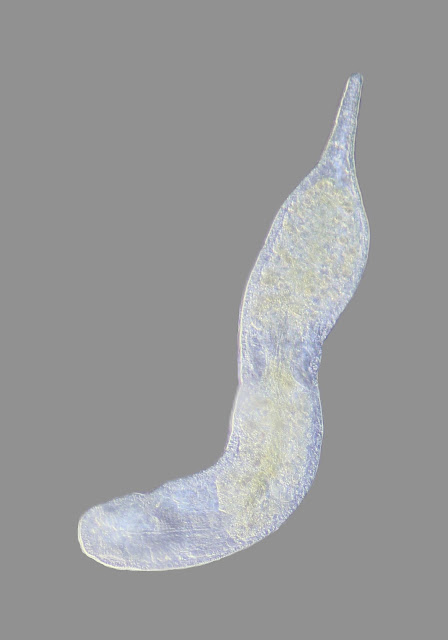 |
| Another view |